
SHARE Recovery Costs for Researchers
You can complete a Feasibility Application Form to request a free search to identify if we have the number of volunteers, samples or data you need for your study, before submitting a Full Application Form. We will send a quote for recruitment, samples or data, once we have received your full application and the SHARE Access/Biobank Committee has approved the study.
SHARE only recovers costs relating to Full Study/ Data/ Biobank applications. We are not running a business. We are funded by the Chief Scientist Office and supported by the Scottish Universities and Health Boards. In order to provide the service, we must recover costs relating to NHS Safe Haven data-linkage, application and recruitment processes. Where a multi-site study is assisted, set up costs can be split between sites.
We only charge a recruitment fee once suitable volunteers are transferred to you and consented to your study.
Should a volunteer be transferred to the study team and subsequently be found to be unsuitable for the study (before screening), the recruitment fee is reduced to a minimal SHARE telephone cost. Please see our cost structure below:
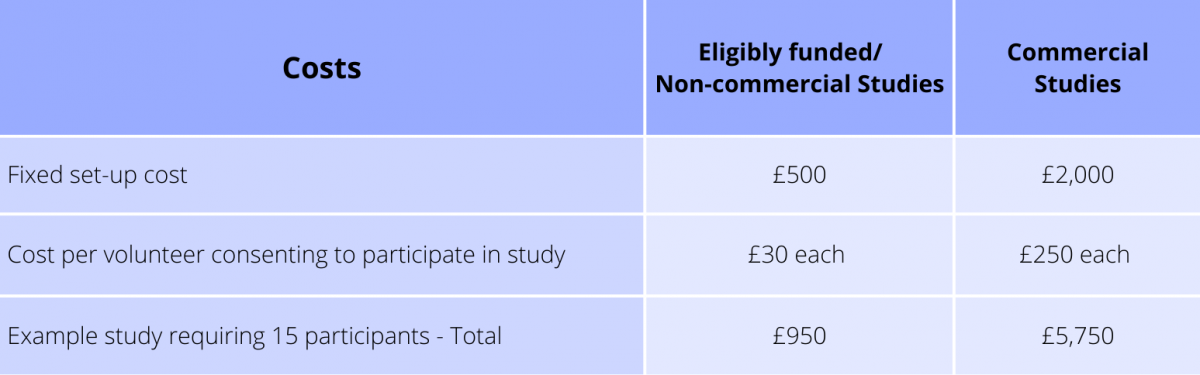
For studies which require genomic information or require anonymised blood samples there is an additional cost for the preparation and sequencing.
Study Costs in Detail
1. A standard Study Set-up fee (£500 for eligibly funded studies; £2,000 for commercial studies)
2. Costs for recruitment (this fee depends on how much work is needed to recruit suitable volunteers)
Study Set-up costs cover:
- Cohort refinement and cohort groups uploaded to the web-based password protected participant tracker application.
- Discussion with the NHS Safe Haven to optimise search.
- Review of, and amendments to the application to ensure fullest and accurate cohort selected.
- Processing of the application (review of application and associated documents: Protocol, NHS R&D and Ethics approvals, Patient Information Leaflet).
- All study-related documents (as above) are sent to SHARE Study Access Committee for consideration and approval.
- Review SHARE’s capability versus eligibility criteria.
- Data linkage (linkage with health records and participant identification).
- Training provided on use of the tracker application. The tracker application can be personalised to help your recruitment process.
Recruitment costs cover (per person):
- Setting up access permissions for web-based tracker application.
- Selection process: potential participants are contacted by the SHARE Study Team (usually by phone). SHARE explains the study and potential participants are asked additional questions (pre-agreed with the study team); this is to identify an accurate cohort.
- Processing of participants on web-based tracker application where the researcher will access participant contact details.
- Participants are passed to the research team at the rate they are able to process and selection refined if required.

 (1).png)

